Our Science
The Unmet Medical Need
Glioblastoma (GBM) is one of the most aggressive and lethal brain cancers, with a median survival of just 14-15 months and a five-year survival rate of less than 10%. Despite the fact that patients receive intense treatment regimens consisting of surgery, radiation, and chemotherapy, recurrence is nearly inevitable and, sadly, most patients eventually succumb to the disease. GBM’s devastating prognosis highlights the urgent need for innovative therapies that can improve survival outcomes and quality of life for patients.

Roughly 15,000 people are diagnosed with glioblastoma in the U.S. every year, accounting for half of all adult brain cancers.

About 12,000 people die of GBM each year in the U.S. – the majority of deaths caused by primary malignant brain tumors.
The Reason: Challenges in Drug Delivery
The challenge of GBM treatment boils down to two things:
First, the blood-brain barrier (BBB) and the blood-brain tumor barrier (BBTB) represent formidable obstacles to effective drug delivery. These barriers restrict over 98% of therapeutic molecules from entering the brain, limiting treatment options to a handful of FDA-approved drugs. Then, to make things worse, even when drugs do cross the BBB, their penetration into the dense and hypoxic GBM tissue remains inadequate. This dual challenge has stymied progress in GBM treatment for decades, leaving patients with limited therapeutic options and poor outcomes.
Our Approach: Drug-Loaded CAR-Neutrophils
We have developed a single, elegant solution that simultaneously overcomes both of these issues and is extremely efficient at delivering highly potent therapeutic compounds deep into glioblastoma tumors: drug-loaded CAR-neutrophils.
We start by engineering human pluripotent stem cells (hPSCs) to express the appropriate chimeric antigen receptor. These are differentiated to produce large quantities of CAR-expressing neutrophils (CAR-NEs) that are then loaded with drug-conjugated nanoparticles and infused intravenously.
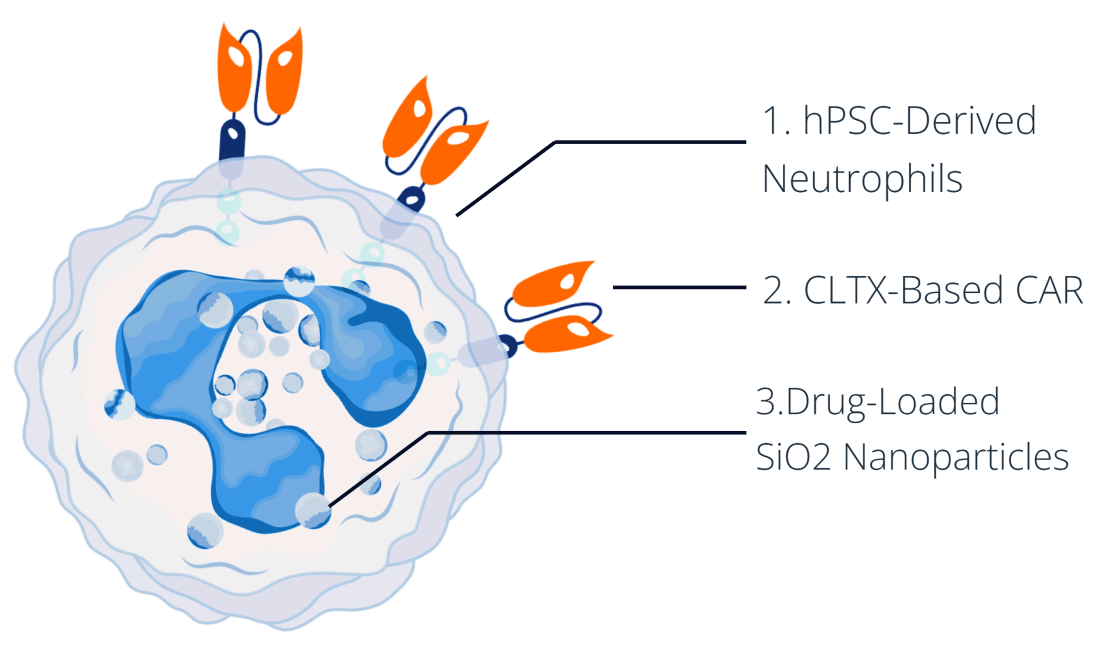
This platform is built on three key components, each playing a critical role in its overall therapeutic effectiveness.
Explore the biological purpose and therapeutic advantages of the three components, below.
1. hPSC-Derived Neutrophils
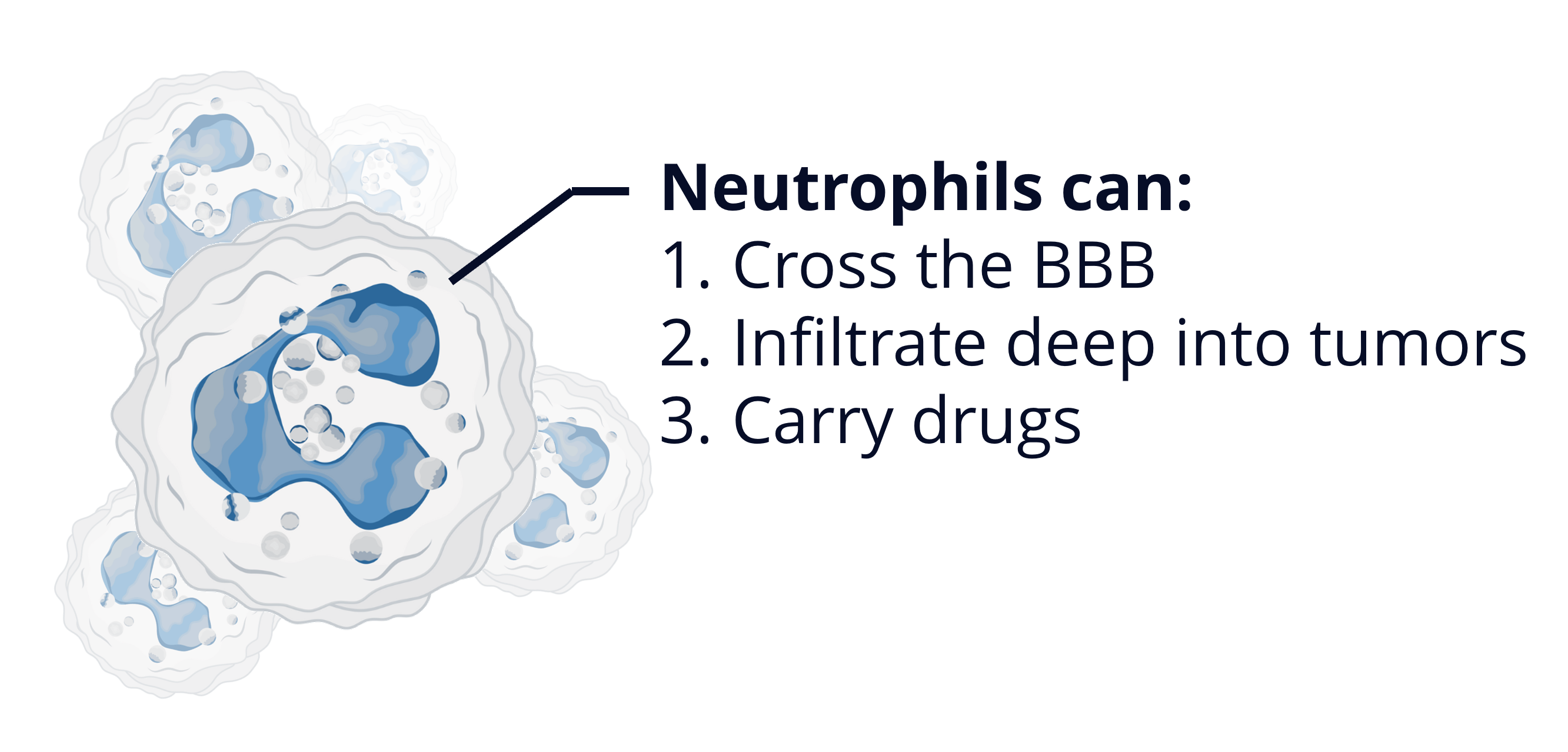
We have chosen to pursue a neutrophil-based drug delivery platform because these cells possess three key characteristics that make them the perfect solution to the challenges facing glioblastoma treatment.
i) Neutrophils naturally possess the ability to cross the BBB.
ii) They are actively recruited into the tumor microenvironment (TME) by inflammatory signals released by cancer cells, allowing them to penetrate deep into glioblastoma tissue.
iii) They can be loaded with therapeutic agents, transforming them into living drug carriers.
This unique combination makes them an ideal platform for delivering anti-cancer drugs to glioblastoma.
2. CLTX-Based Chimeric Antigen Receptor
While it is true that neutrophils harbor many therapeutically advantageous characteristics, they have a pretty significant drawback:
Once inside the tumor, they tend to become highly immunosuppressive and instead of killing cancer cells, begin to help the cancer grow.
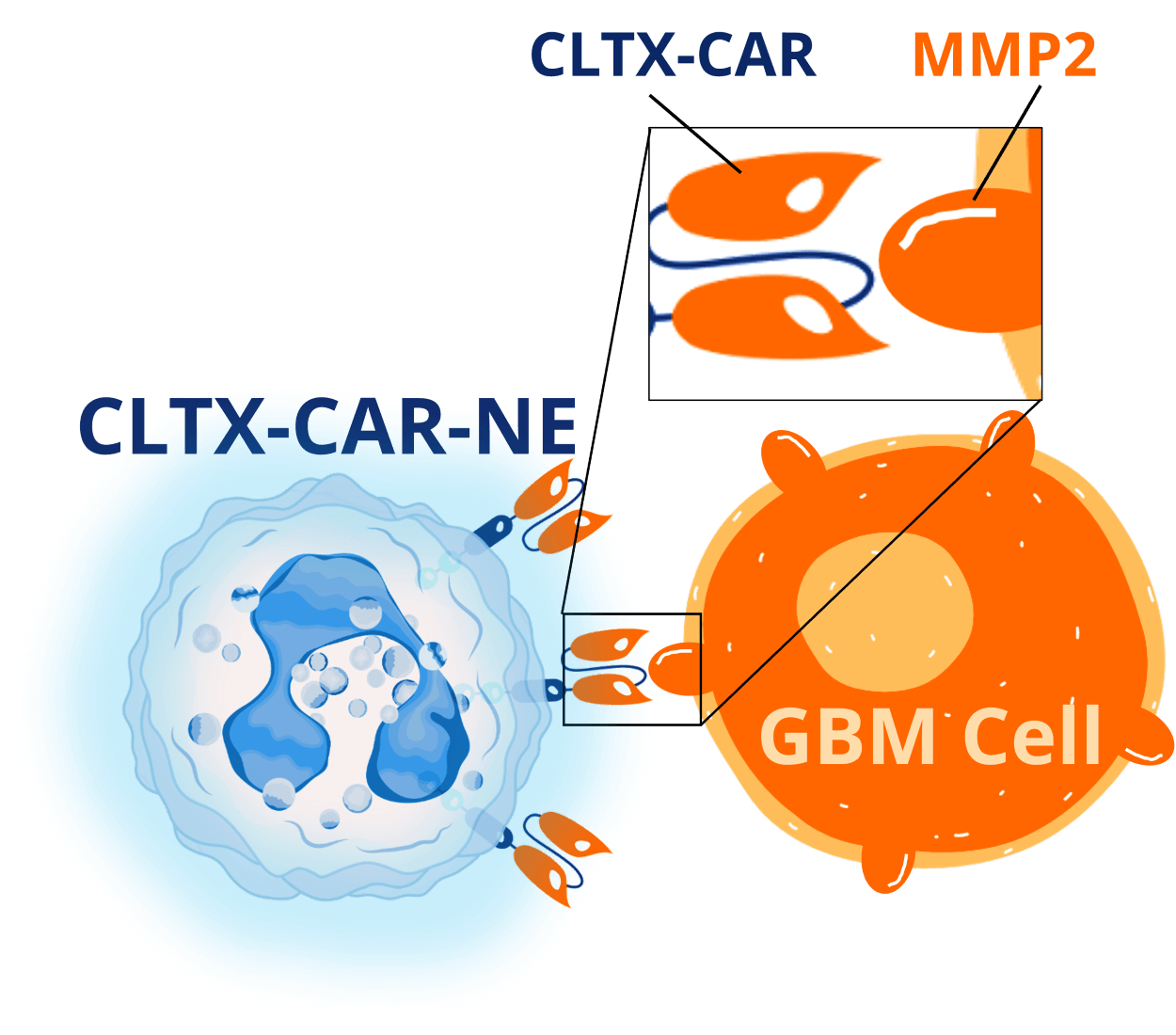
This is obviously not good and poses a major hurdle to using them as an anti-cancer therapy. So, to prevent this, we express a chlorotoxin-based CAR on our neutrophils – these are termed CLTX-CAR-NEs.
CLTX binds to MMP2, which is highly expressed on the surface of many GBM cells. When the CLTX-CAR-NEs get inside the tumor, the CAR will bind to MMP2-positive GBM cells, send stimulatory signals to the neutrophil, and prevent it from becoming immunosuppressive.
Our preclinical data has shown that the CLTX-CAR is essential for maintaining the anti-cancer functionality of the neutrophils.
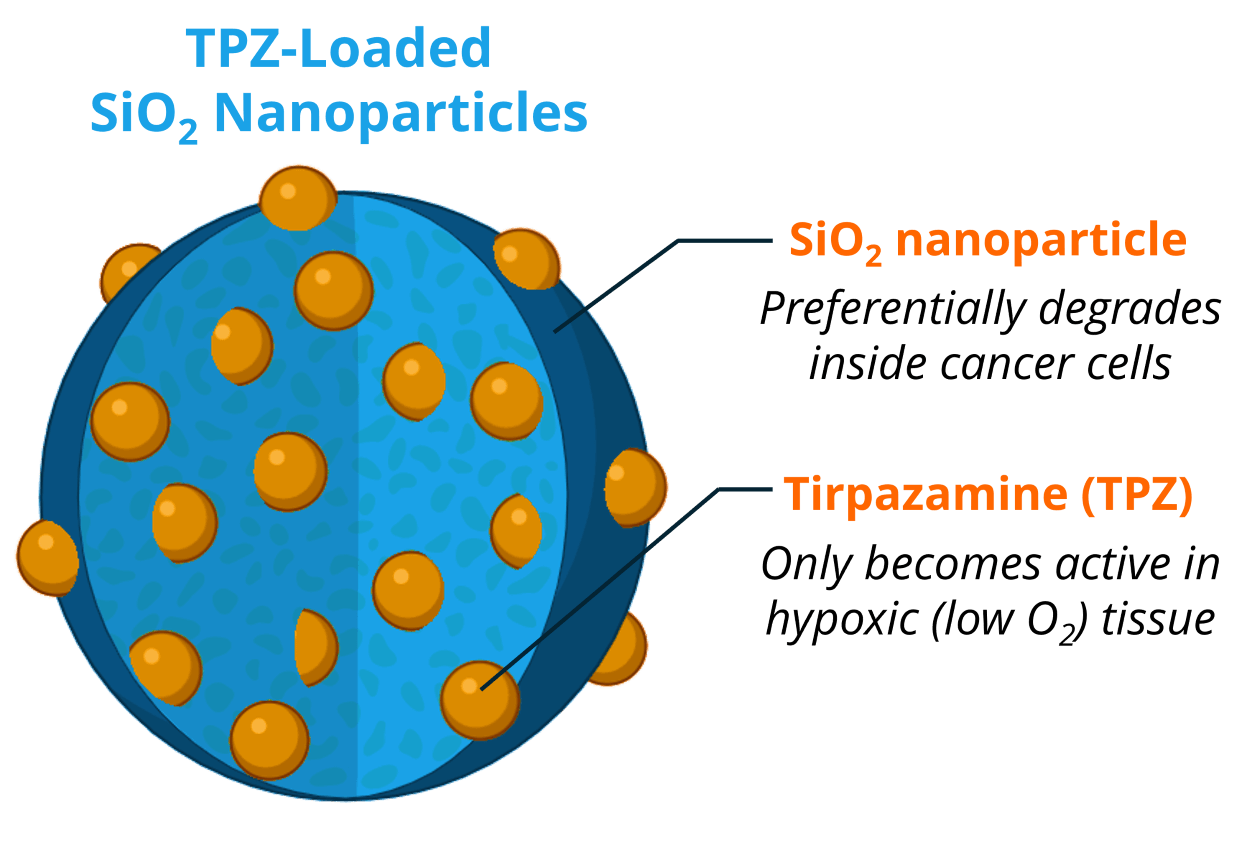
3. TPZ-Loaded SiO2 Nanoparticles
We have strategically chosen to use Tirapazamine (TPZ)-loaded SiO2 nanoparticles for two reasons:
i) SiO2 nanoparticles preferentially degrade within cancer cells due to the high levels of glutathione. This way, even if the nanoparticles get into a non-cancerous cell, the drug remains encapsulated and won’t harm the healthy cell.
ii) TPZ is a “hypoxia-induced prodrug” meaning that it only becomes active in low-oxygen conditions – a very common characteristic of GBM. So, even if the nanoparticles were to get into healthy tissue, and somehow release TPZ, the drug wouldn’t be active, further reducing the potential for off-target toxicities.
This highly strategic design makes this therapeutic incredibly safe to healthy cells while selectively targeting hypoxic GBM tissue.
The Result: A Highly Potent and Safe Cell-Based Therapy for GBM
Together, these three components synergize to produce a highly potent cell-based drug delivery platform that is designed to selectively target deep-seeded hypoxic GBM tissue.
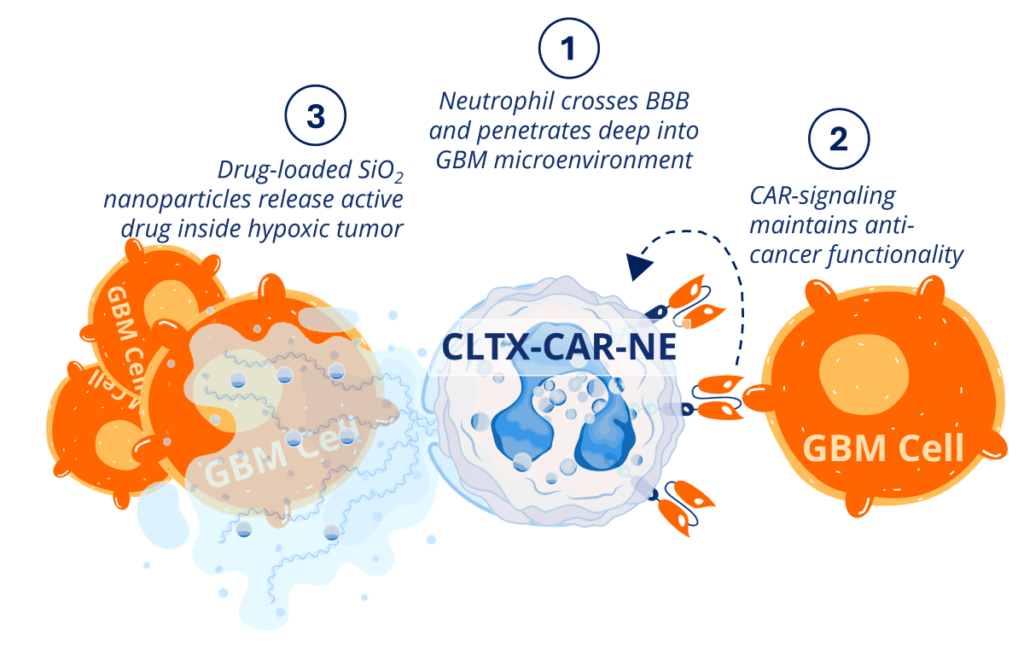
Inside the body, our drug-loaded CAR-NEs cross the BBB and infiltrate deep into the core of glioblastoma tumors. Once inside, CAR-NEs behave like any normal neutrophil, targeting and killing cancer cells through a variety of mechanisms. Then, when they die, they release their cytotoxic payload, inducing incredibly potent anti-cancer effects with very little risk of toxicities.
Our Cell Generation Platform
Our cell therapy platform integrates advanced stem cell technology, genetic engineering, and nanotechnology to create a highly effective drug delivery system for glioblastoma.
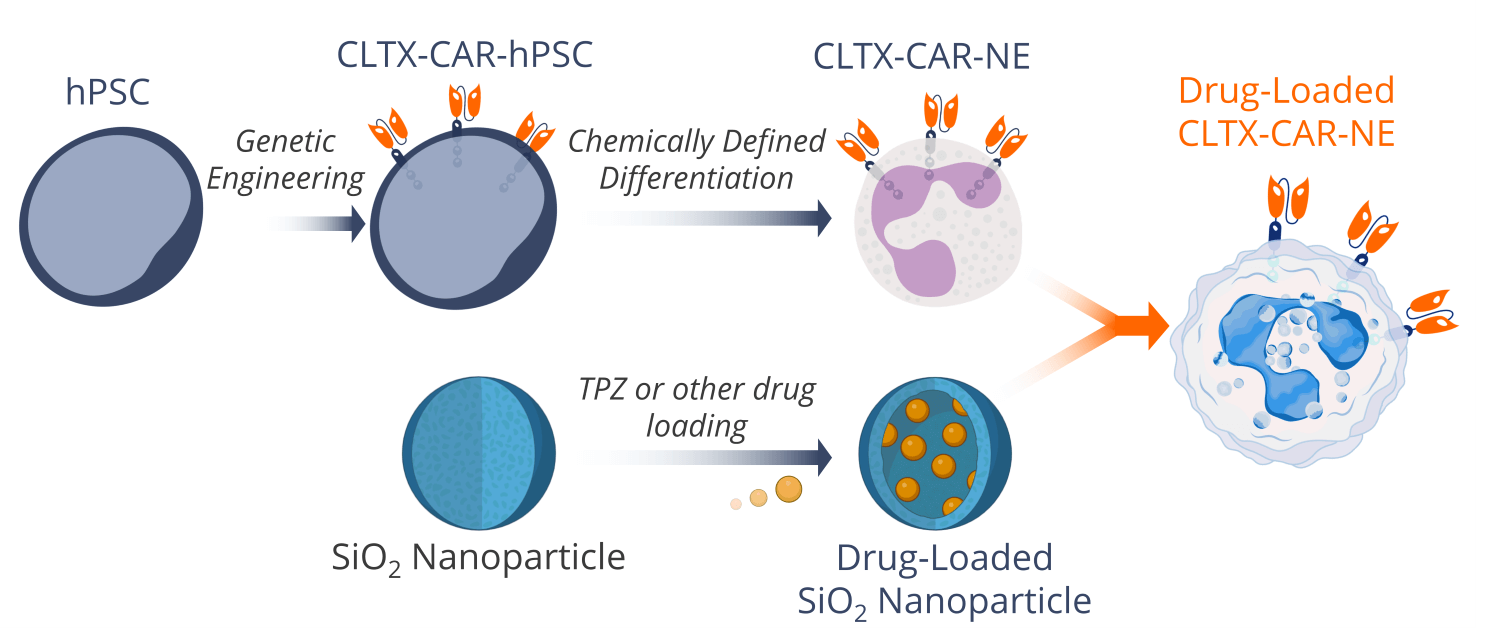
We start with human pluripotent stem cells (hPSCs) that we then genetically engineer to express a chlorotoxin-based chimeric antigen receptor (CLTX-CAR) for tumor specificity and anti-cancer functionality. These engineered hPSCs are then differentiated into CAR-neutrophils using a proprietary, chemically-defined differentiation protocol.
Simultaneously, silicon oxide (SiO2) nanoparticles are loaded with tirapazamine (TPZ) (it is possible to load other therapeutic compounds here as well). Once the neutrophils are fully differentiated, they readily phagocytose the SiO2 nanoparticles, transforming into living drug carriers capable of crossing the blood-brain barrier and delivering therapeutics deep into tumor tissue.
Invest in Infinova
As an investor, you’ll have the opportunity to support a paradigm-shifting therapeutic approach that addresses critical unmet needs, offering hope to patients and significant potential for impact. Together, we can drive innovation, advance science, and make a lasting difference in the fight against one of the most devastating cancers.
Useful Links
About Us
Learn about our mission, discover the philosophy at the heart of our therapeutic approach, and meet the Infinova team.
News and Media
Check out the media center to keep up with our latest news and our upcoming events.
Contact Us
Feel free to reach out and ask any questions or express any concerns. We believe in open dialogue.